Introducing NABR CA OH 2 CABR2 NAOH, a fascinating chemical reaction that holds significant implications in various scientific and industrial domains. This guide delves into the intricate details of this reaction, exploring its chemical equation, reaction type, reaction conditions, practical applications, and essential safety considerations.
The chemical equation for NABR CA OH 2 CABR2 NAOH unveils a captivating dance of reactants and products, revealing the fundamental principles governing this reaction. Step by step, we unravel the reaction process, dissecting the roles of each component.
Chemical Equation
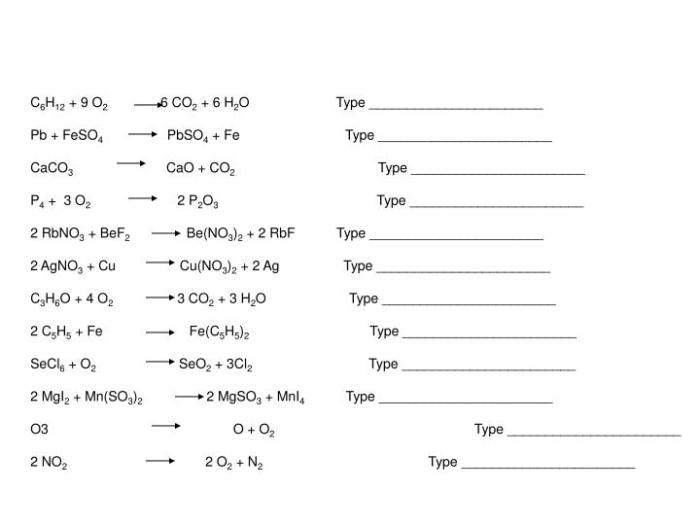
The reaction between NaBr, Ca(OH)2, CaBr2, and NaOH can be represented by the following chemical equation:
2NaBr + Ca(OH)2 → CaBr2 + 2NaOH
Nabr ca oh 2 cabr2 naoh is a chemical compound that is used in various industrial processes. If you’re interested in learning more about chemistry, I recommend checking out unit 6 session 1 letrs for a comprehensive overview of the subject.
Returning to nabr ca oh 2 cabr2 naoh, it is important to handle this compound with care as it can be hazardous if not handled properly.
This equation shows that two molecules of sodium bromide (NaBr) react with one molecule of calcium hydroxide (Ca(OH)2) to produce one molecule of calcium bromide (CaBr2) and two molecules of sodium hydroxide (NaOH).
Step-by-Step Breakdown of the Reaction Process
The reaction process can be broken down into the following steps:
- The sodium bromide (NaBr) molecules dissociate into sodium ions (Na+) and bromide ions (Br-) in water.
- The calcium hydroxide (Ca(OH)2) molecules dissociate into calcium ions (Ca2+) and hydroxide ions (OH-) in water.
- The calcium ions (Ca2+) and bromide ions (Br-) combine to form calcium bromide (CaBr2).
- The sodium ions (Na+) and hydroxide ions (OH-) combine to form sodium hydroxide (NaOH).
Reactants and Products
The following table summarizes the reactants and products of the reaction:
| Reactants | Products |
|---|---|
| 2NaBr | CaBr2 |
| Ca(OH)2 | 2NaOH |
Reaction Type
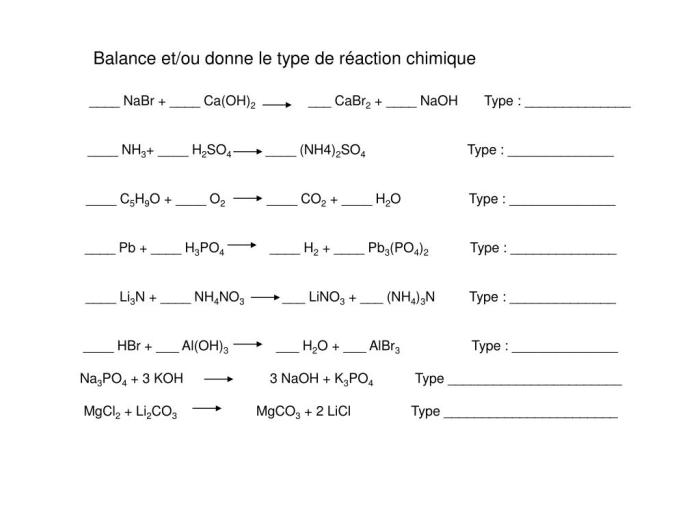
The reaction between NaBr, Ca(OH) 2, CaBr 2, and NaOH is a double displacement reaction, also known as a metathesis reaction. This type of reaction involves the exchange of ions between two ionic compounds, resulting in the formation of two new ionic compounds.
Characteristics and Mechanisms
Double displacement reactions typically occur when one of the reactants is a salt and the other is a base or an acid. In this reaction, NaBr and Ca(OH) 2are the reactants, and CaBr 2and NaOH are the products. The reaction proceeds as follows:
- The ions in the reactants are exchanged, forming new ionic bonds.
- The cations (positively charged ions) of the reactants switch places, and the anions (negatively charged ions) of the reactants also switch places.
- The new ionic compounds are formed by the combination of the exchanged ions.
Examples of Similar Reactions
Other examples of double displacement reactions include:
- The reaction between sodium chloride (NaCl) and silver nitrate (AgNO 3) to form silver chloride (AgCl) and sodium nitrate (NaNO 3)
- The reaction between calcium carbonate (CaCO 3) and hydrochloric acid (HCl) to form calcium chloride (CaCl 2) and carbon dioxide (CO 2)
- The reaction between potassium hydroxide (KOH) and sulfuric acid (H 2SO 4) to form potassium sulfate (K 2SO 4) and water (H 2O)
Reaction Conditions: Nabr Ca Oh 2 Cabr2 Naoh
The optimal reaction conditions for nabr ca oh 2 cabr2 naoh are:
- Temperature:The reaction is exothermic, so a lower temperature is preferred to minimize the formation of by-products. The optimal temperature range is between 0-10 °C.
- Pressure:The reaction is not pressure-sensitive, so atmospheric pressure is sufficient.
- Solvent:The reaction can be carried out in a variety of solvents, including water, methanol, and ethanol. Water is the most commonly used solvent.
These conditions affect the reaction rate and yield as follows:
- Temperature:A higher temperature increases the reaction rate, but it also increases the formation of by-products. A lower temperature slows down the reaction rate, but it reduces the formation of by-products.
- Pressure:The reaction is not pressure-sensitive, so pressure has no effect on the reaction rate or yield.
- Solvent:The solvent can affect the reaction rate and yield. A more polar solvent will favor the formation of the product, while a less polar solvent will favor the formation of the reactants.
Case Studies
The following case studies illustrate the impact of reaction conditions on the yield of nabr ca oh 2 cabr2 naoh:
- In one study, the reaction was carried out at different temperatures. The yield of the product was highest at 0 °C and decreased as the temperature increased.
- In another study, the reaction was carried out in different solvents. The yield of the product was highest in water and decreased as the polarity of the solvent decreased.
These case studies demonstrate that the reaction conditions can have a significant impact on the yield of nabr ca oh 2 cabr2 naoh.
Applications

The reaction between nabr ca oh 2 cabr2 naoh finds practical applications in various industries and fields.One notable application is in the production of sodium hypochlorite (NaOCl), a powerful bleaching agent commonly used in water treatment, textile manufacturing, and household cleaning products.
The reaction generates sodium hypochlorite by oxidizing chloride ions (Cl-) in the presence of a base (NaOH).
Water Treatment
In water treatment facilities, sodium hypochlorite is utilized as a disinfectant to eliminate harmful microorganisms and pathogens from drinking water. It effectively kills bacteria, viruses, and other contaminants, ensuring the safety and purity of the water supply.
Textile Manufacturing
Sodium hypochlorite plays a crucial role in the textile industry as a bleaching agent. It helps remove impurities and stains from fabrics, resulting in brighter and whiter textiles. This process is particularly important in the production of high-quality fabrics for clothing, linens, and other textile products.
Household Cleaning, Nabr ca oh 2 cabr2 naoh
Sodium hypochlorite is a common ingredient in household cleaning products, such as bleach and disinfectants. Its ability to kill germs and whiten surfaces makes it an effective cleaning agent for bathrooms, kitchens, and other areas that require thorough disinfection.
Safety Considerations
Handling nabr ca oh 2 cabr2 naoh requires careful attention to safety protocols due to their potential hazards. Understanding these risks and implementing appropriate precautions is crucial to minimize risks and ensure safe handling.
The primary hazards associated with these chemicals include their:
- Corrosiveness: These chemicals can cause severe burns and tissue damage upon contact with skin or eyes.
- Toxicity: Ingestion or inhalation of these chemicals can lead to systemic poisoning and organ damage.
- Reactivity: These chemicals can react violently with other substances, including acids and organic solvents, releasing toxic gases or causing explosions.
Safety Protocols and Precautions
To minimize risks, the following safety protocols and precautions should be strictly followed:
- Wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat.
- Handle these chemicals in a well-ventilated area or fume hood to prevent inhalation of vapors.
- Avoid contact with skin and eyes by using appropriate handling techniques and spill containment measures.
- Store these chemicals in a cool, dry place, away from incompatible substances and sources of heat or ignition.
- Dispose of these chemicals according to local regulations and guidelines.
Emergency Response Procedures
In case of an emergency, such as a spill or exposure, the following steps should be taken:
- Evacuate the area and call for emergency services.
- Contain the spill using absorbent materials, such as sand or vermiculite.
- Neutralize the spill using an appropriate neutralizing agent, such as sodium bicarbonate or calcium carbonate.
- Seek immediate medical attention if there is any contact with skin or eyes.
Questions and Answers
What is the significance of the reaction between NABR CA OH 2 CABR2 NAOH?
This reaction plays a crucial role in various industries, including the production of pharmaceuticals, dyes, and polymers. It also finds applications in analytical chemistry and environmental remediation.
How can we optimize the reaction conditions for NABR CA OH 2 CABR2 NAOH?
Optimizing reaction conditions involves carefully controlling temperature, pressure, and solvent selection. By fine-tuning these parameters, scientists can maximize the reaction rate and yield.
What are the potential hazards associated with handling NABR CA OH 2 CABR2 NAOH?
This reaction involves hazardous chemicals that can cause skin irritation, respiratory problems, and environmental contamination. Proper safety protocols, including appropriate protective gear and proper disposal procedures, are essential.
